Viruses in the Brain - Part I: Complement
TLDR: The complement system is both part of the immune system and a player in influencing normal brain function/connectivity. Activation of the complement system in the brain during virus infections leads to cognitive issues. Part of a broader picture of how viruses and inflammation can contribute to neurodegenerative processes.
.png)
This is part I of a series on one of my major research focuses—what happens when viruses get inside the brain.
Viruses make their way into our brains—likely more often than we detect. Impairments in our ability to think and remember are a widely reported phenomena after brain infections. In the most extreme cases, severe infections can alter a person’s behaviors and personality, fundamentally changing who they are. After all, viruses are some of the most elegant deliverers of foreign genes. Both the expression of viral genes and the cellular response to viral infection can negatively impact cells in the brain and consequently our higher-order processes (thinking, sensing, feeling).
Here, we have a situation where one constellation of proteins (the complement system) is used for two different functions in the body. We’ll see how this causes conflict when those two functions collide in one environment: the virus-infected brain.
What’s Complement?
The complement system is but one component of the immune system also located in the brain. It is a series of secreted proteins that cleave other proteins, amplifying signal in a fashion similar to conventional, intracellular signaling cascades of cell surface receptors. This pathway is ancient, huge, and convoluted. The huge number of involved proteins is additionally complicated by multiple cleaved forms (requiring targeting of distinct epitopes for antibody-based detection methods), differences between animal models and humans, and complex genetic organization (see the human C4 locus).
Complement was first discovered in the context of blood and pathogen defense. If you encounter it in medical school curriculum, it makes brief appearances in immunology and kidney pathology. Very generally, activated complement sticks to things. It acts to tag pathogens, spurring inflammation or targeting them for destruction by downstream processes (phagocytosis or the membrane attack complex). One metaphor is complement as a sonar system. Complement has a homeostatic level of continuous turnover (termed tick-over) which tags both diseased and un-diseased cells but the balance between activation and inhibition is mediated by a bunch of surface-bound complement regulators (and a few soluble ones). Again, complement is activated by many pathogens, which facilitates the elimination of the pathogen either directly or by plugging into other inflammatory responses.
Complement in the Brain
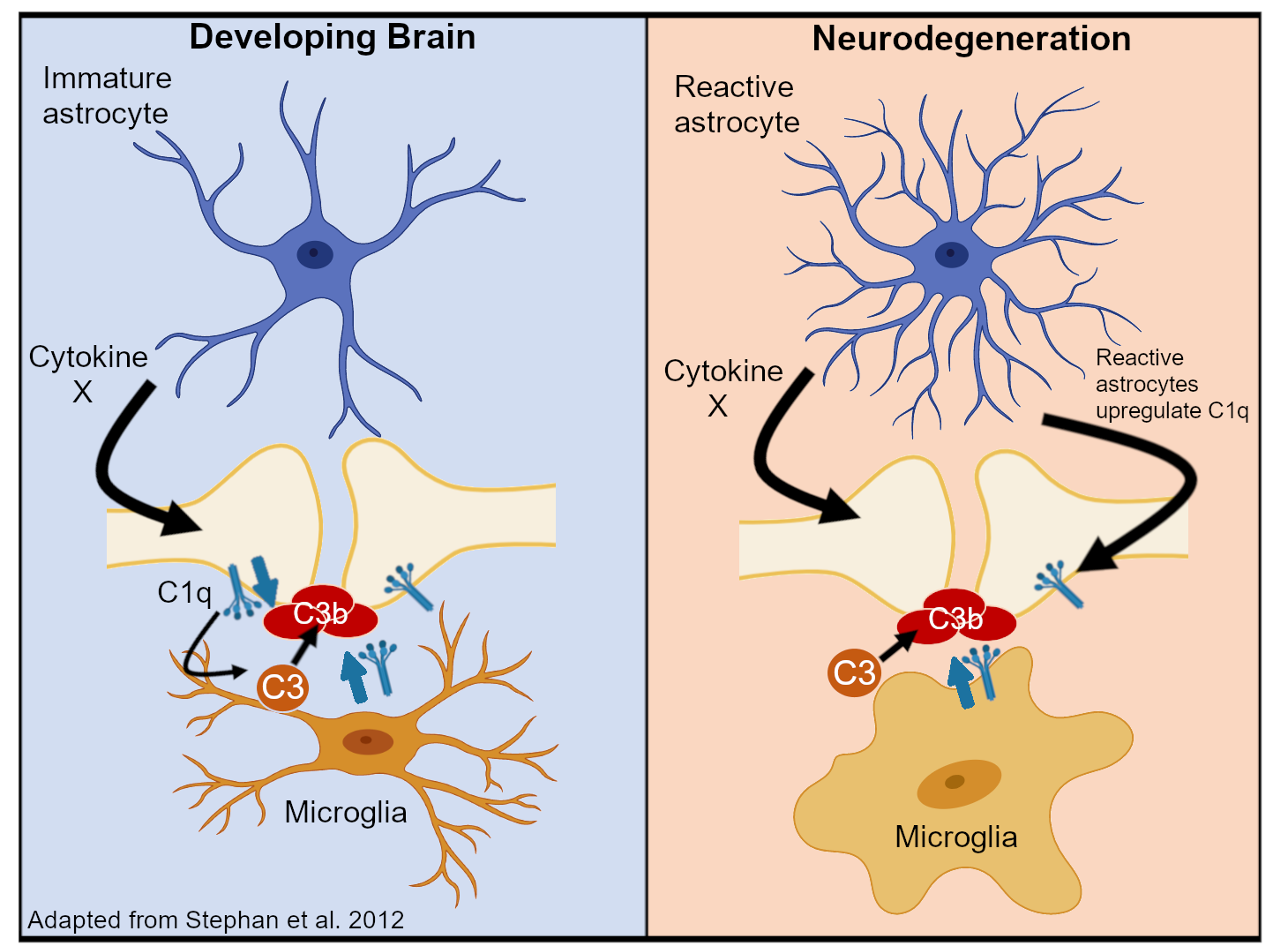
However, this system is also involved in neurodevelopment and elimination of synaptic connections. Specifically, the complement system has a role in determining the final positioning of certain cells in the brain and in refining the circuitry of the brain. The first is linked to concentration-dependent gradients much like the morphogens that pattern the body.
The second is more tied to the classic model of opsonization with bound complement acting as a tag for phagocytic cells. Early in life, the brain will tend towards over-connecting neurons by synapses. Synapses are generally preserved based on their activity—a “use it or lose it” scenario. Throughout the life course, excess connections are pruned away at variable rates. Microglia and complement are involved in this process of clearing away these connections. As this network of connections relates to memory, complement is experimentally linked to the active process of forgetting. A paper from Drs. Lang Wang and Yan Gu’s labs demonstrated promotion or inhibition of ‘forgetting’ in mice by messing with complement, mechanistically linking the two.
Besides the ‘physiologic’ roles of complement, complement also plays a part in a huge number of neurodegenerative and neuroinflammatory diseases. The list is extensive including Alzheimer’s, trauma, and schizophrenia. In these conditions, deposition of activated complement proteins is related to axonal damage (part of those synaptic connections) and cell death.
Brain + Complement + Virus
When a pathogen reaches the brain, these dual functions of the complement system—its roles in pathogen immunity and brain homeostasis—collide. What happens when these two distinct contexts occur in a single environment (the brain)?
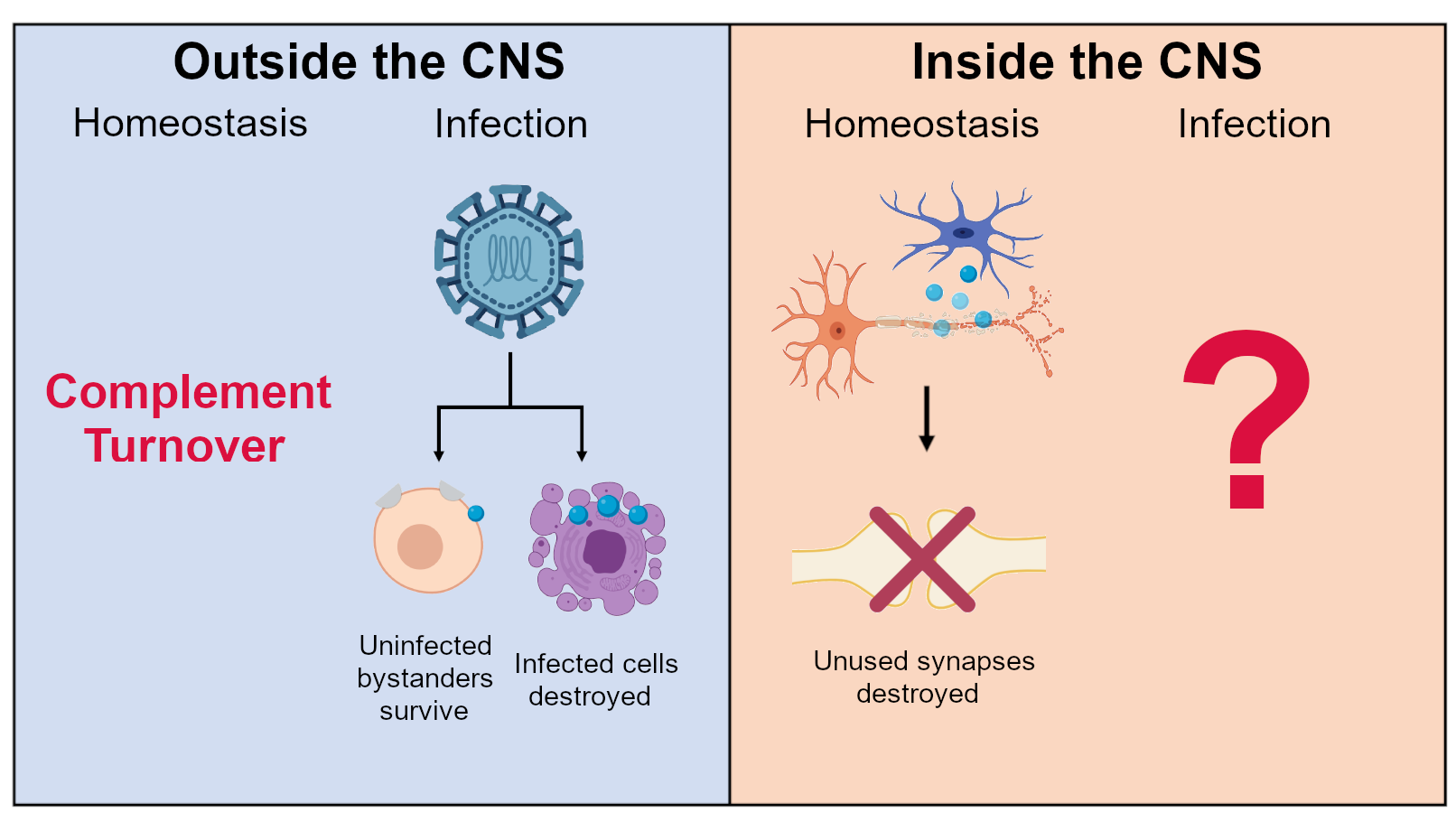
There are a bunch of ways brain, complement, and virus interact. For pathogens that travel through the blood to reach the brain (arboviruses like Zika virus for example), these microbes often encode proteins that can affect the complement system. Circumventing the complement system is a highly conserved mechanism for intra-host dissemination through inactivation or sequestration of complement-related proteins. By messing up when or where complement proteins bind, viral proteins could affect the downstream consequences of the cascade.
As interferon-stimulated genes, profound changes to the expression of some complement-related proteins are found upon infection. The most prominent paper linking the complement system and a flavivirus in the brain was a collaboration between Drs. Robin Klein’s and Beth Stevens’ labs. This paper looked at West Nile virus in mice and found that complement deposition, particularly of the complement protein C3, was increased and contributed to memory deficits.
My master’s thesis sought to find similar interactions between a different flavivirus, Zika virus, and the complement system in the brain. I joined the lab of Dr. Ernesto Marques who was extremely involved in Brazil’s response to Zika and investigated ties to microcephaly. Zika could potentially impact the complement system at two points in human development: in utero after Zika crosses the placenta or after birth. Decades of research (mostly by the Dr. Michael Diamond lab) also indicated that a flaviviral protein NS1 could interact directly with the complement system by binding many of the involved proteins. How to study the effects of the virus and its proteins on human brains?
Around this time, organoid technology had debuted with the emergence of human cortical organoids. A collaboration that involved the Drs. Guo-Li Ming, Hongjun Song, and Pei-Yong Shi labs (where I currently work now) demonstrated the utility of organoids in modeling neuroinfectious disease. In Dr. Ernesto Marques’ lab, I collaborated with Dr. Vishwajit Nimgaonkar and Dr. Leonardo D’Aiuto, who were applying these models to Zika and herpes simplex virus 1. In terms of resources, we had established assays (ELISAs) to measure complement protein levels for past flavivirus work and initially applied them to samples from infected and uninfected organoids. We quickly found that levels of complement proteins were extremely low or zero in the supernatant from these cells. Our first step was to figure out how to get the full range of complement in vitro.
Outside the brain, most complement comes from the liver. We thought there might be some leakage of blood and consequently complement into the brain and we considered supplementing the cell media with the proteins in fixed ratios. However, early work by Dr. Paul Morgan’s group (late 90’s - early 00’s) indicated production of complement proteins by brain cells themselves. We had to figure out the right combination of cells and establish which cell types produce what complement protein.
While it was ambiguous at the time, we now know that neurons, astrocytes, and microglia are all important producers of complement proteins, especially when cultured together. Single-cell sequencing demonstrated upregulation of complement transcripts (usually C1q, C3, and C4) in pro-inflammatory states. Eventually, an analogous system involving iPSC-derived cells was created (described here) but I never got to apply similar principles to this system (the COVID pandemic started). While there are currently few reported organoid models with demonstrably ‘complete’ complement systems, most systems that include neurons, astrocytes, and microglia should have these proteins in culture.
At the time, there was an underappreciation for complement inhibitors or regulators in the brain complement literature. Many studies demonstrated C3 deposition on neurons with immunostaining and assumed overactivation was mostly due to excessive production rather than under-regulation. Excessive production is indeed supported by transcriptional increases of complement proteins in inflammatory neuron, astrocyte, and microglia phenotypes but it’s still likely the balance between effectors and regulators that determines complement-mediated outcomes.
Naturally, understanding whether infections tip this balance is important. With numerous scientific studies tying viruses and neurodegenerative diseases like Alzheimer’s together, the viruses residing in our brains could affect regulation and production of complement, perhaps not triggering but contributing to the pathology of these conditions.
SARS-CoV-2 and Brain Complement
Now, the obvious relevance to COVID-19. In light of neuro-COVID and long COVID (specifically symptoms like brain fog), is there a role for brain complement?
For severe systemic disease, pronounced complement activation is really well established. In this setting, the complement system also links to the coagulation cascade, driving clot formation and vascular damage. In this way, SARS-CoV-2 activation of complement could be tied to one prominent hypothesis for long COVID symptoms: the formation of clots (especially micro-clots).
If you look in the neuro-COVID literature, complement pops up a fair bit and the connection has been drawn in several review articles. One of the most prominent papers relating to complement came from Dr. Avindra Nath’s group, tying post-infectious changes in coagulation, complement, and brain inflammation together. You’ll also see high levels of complement (usually C3 and C1q) in papers studying the brains of people who died from severe COVID or the blood of long COVID subpopulations. Finally, the same role in synaptic pruning has been implicated in SARS-CoV-2, much like the flavivirus experiments. While the existence of a persistent SARS-CoV-2 reservoir in the brain is still disputed, the observed neuroinflammatory changes suggest complement activation can occur even without active, replicating virus. Overall, the brain complement work for SARS-CoV-2 largely mirrors what we know for other viruses with some descriptions of unique interactions.
One advantage of targeting the complement system is the large number of potential drug targets. There are several drugs that can target different parts of its many pathways. For COVID-19, small, promising trials have been performed but the fate of these drugs is now uncertain given lower numbers of severe cases.
Anti-complement drugs are being trialed in non-virus-related neurologic conditions. Targeting complement in viral CNS infection has been explored in animal models but not in human trials. Ultimately, it is still unclear if inhibiting complement activation in the brain after certain developmental stages has any deleterious effects although existing systemic complement inhibitors have clear side effects outside the brain. Across several neurodegenerative conditions, complement inhibition improves outcomes. In the context of infection, inhibition at certain stages could prevent viral clearance if brain complement plugs into downstream innate and adaptive signaling like it does in the periphery (I haven’t seen compelling evidence that brain complement participates directly in antiviral defense). However, the brain complement field is rapidly growing. With the established importance to a wide variety of disorders, I am sure we will see exciting translational work from this field.

MD/PhD Student at University of Texas Medical Branch
I write about viruses, data science, and ballet.
Click home at the top of the page to see other things I’ve been working on.